By Professor Luis E. C. Andrade, Rheumatology Division, São Paulo Federal University, São Paulo, Brazil
This is a project of the Autoantibody Standardization Committee and the International Consensus on ANA Patterns (ICAP), under the Quality Assessment and Standardization Committee of the International Union of Immunology Societies (IUIS).
Autoantibodies are a hallmark of systemic autoimmune diseases and the indirect immunofluorescence assay on HEp-2 cells (HEp-2 IFA), historically known as the antinuclear antibodies (ANA) test, is usually performed as part of the initial diagnostic workup in these patients. HEp-2 IFA offers preliminary information on the possible autoantibodies in a given sample, according to the morphological immunofluorescence patterns. Since 2014, the International Consensus on ANA Patterns (ICAP), an IUIS initiative, has developed a consensus classification of the most consistent HEp-2 IFA patterns, with representative images and detailed information available at www.anapatterns.org.
Despite the wide acceptance of ICAP recommendations by clinical laboratories, academic institutions and in vitro diagnostic industries, it is generally recognized that there is considerable heterogeneity across the world regarding the interpretation and reporting of HEp-2 IFA. The scientific publications regarding the prevalence of HEp-2 IFA are most frequently based on local/regional data, making it difficult to accurately register possible differences in the frequency of positive HEp-2 IFA and of each HEp-2 IFA pattern in different parts of the world. The HEp-2 CIC project aims to contribute to fill these gaps, by determining the frequency of positive HEp-2 IFA and the respective patterns in laboratories worldwide.
Among the 30 HEp-2 IFA patterns classified by ICAP, some are not entirely characterized in terms of autoantibody association and clinical relevance. In addition, some rare HEp-2 IFA patterns have no consistent immunologic and clinical characterization, and do not appear in the ICAP classification algorithm. Single research centres have difficulty in gathering a sufficient number of cases necessary for definitive characterization.
With these considerations in mind, ICAP has launched the HEp-2 CIC project, with three independent branches:
1) Determination of the reporting characteristics of HEp-2 IFA ICAP patterns worldwide
2) Establishment of clinical correlations of selected HEp-2 IFA patterns
3) Characterization of the antigenic specificity of selected HEp-2 IFA patterns
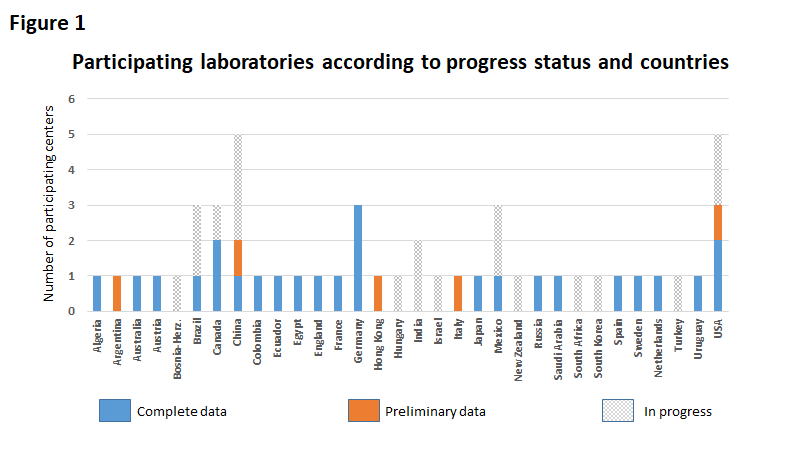
While the second and third branches are at the stage of planning and structuring, the first branch has progressed considerably, with participation of 47 laboratories form 32 countries in five continents (Figure 1). Each laboratory contributes the data from the HEp-2 IFA of all patients during a one-year period. In addition to the geographic comprehensiveness, the study embraces private/public and general/specialty laboratories. There are large and small laboratories, ranging from 5,000 to over 200,000 HEp-2 IFA assays per year. Preliminary analyses of partial data confirms the expectation of considerable heterogeneity in recognition and reporting of the HEp-2 IFA pattern. For example, the dense fine speckled nuclear pattern (Figure 2A) is strongly associated to anti-DFS70 antibodies and is considered a robust indication of absence of systemic autoimmunity. As shown in Figure 2B, participating centers in different countries vary considerably with respect to reporting this pattern. A similar situation has been observed for several other patterns. These differences possibly indicate heterogeneity in the way analysts and investigators interpret the HEp-2 IFA test in different parts of the world. However, regional differences in the prevalent autoantibody profiles may also contribute to this phenomenon. The differences identified in the HEp-2 IFA project will serve as starting point for further action in the international harmonization of the HEp-2 IFA test and for studies investigating possible environmental/ethnical effects on the prevalence of autoantibodies in different parts of the world.